Home
electronic Self-Administered Psoriasis Area and Severity Index
eSA-PASI
Psoriasis self-assessment made
easy and quick
Illustrative PDF
(coming soon)
Original PDF
(coming soon)
What is the eSA-PASI?
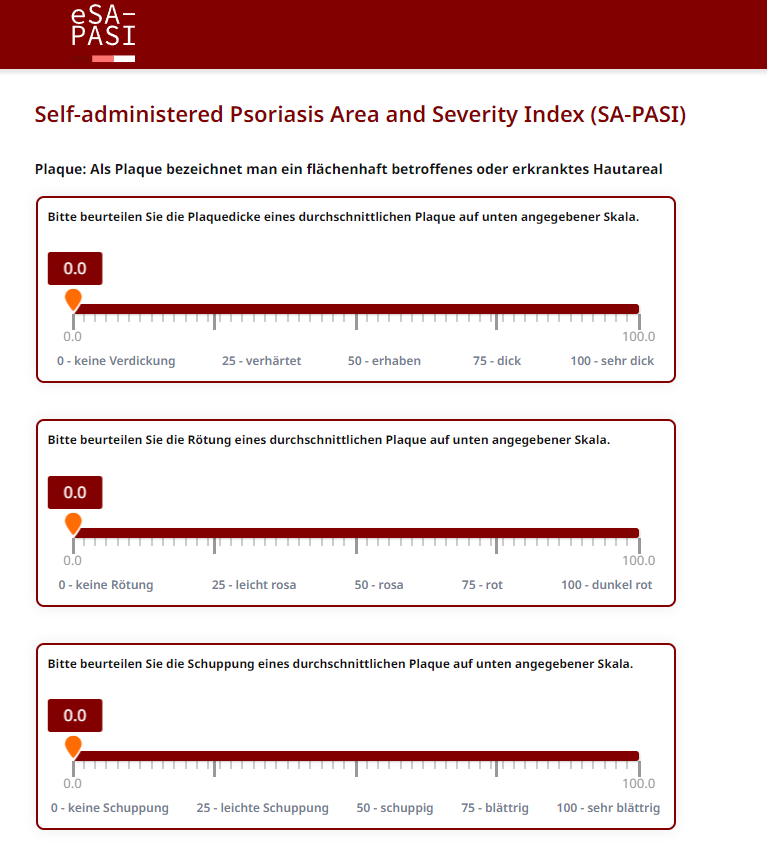
The electronic Self-Administered Psoriasis Area and Severity Index (eSA-PASI) is intended to measure the psoriasis severity by patients themselves. The SA-PASI evaluates the area affected by psoriasis and captures its redness, thickness and scaliness. A regular SA-PASI assessment may help to continuously monitor the progression of the disease.
Validation of questionnaire
paper version: yes digital version: no
How to use the eSA-PASI
The questionnaire is self-explanatory and consists out of two parts. In the first part, three questions help to assess the severity of a typical psoriasis plaque based on thickness, redness and scaling. The second part determines the extent of the body surface affected by psoriasis.
Below is an example of the eSA-PASI assessment process in two steps.
In the first part, the severity of psoriasis symptoms is recorded by assessing the redness, thickness and scaling of the plaques.
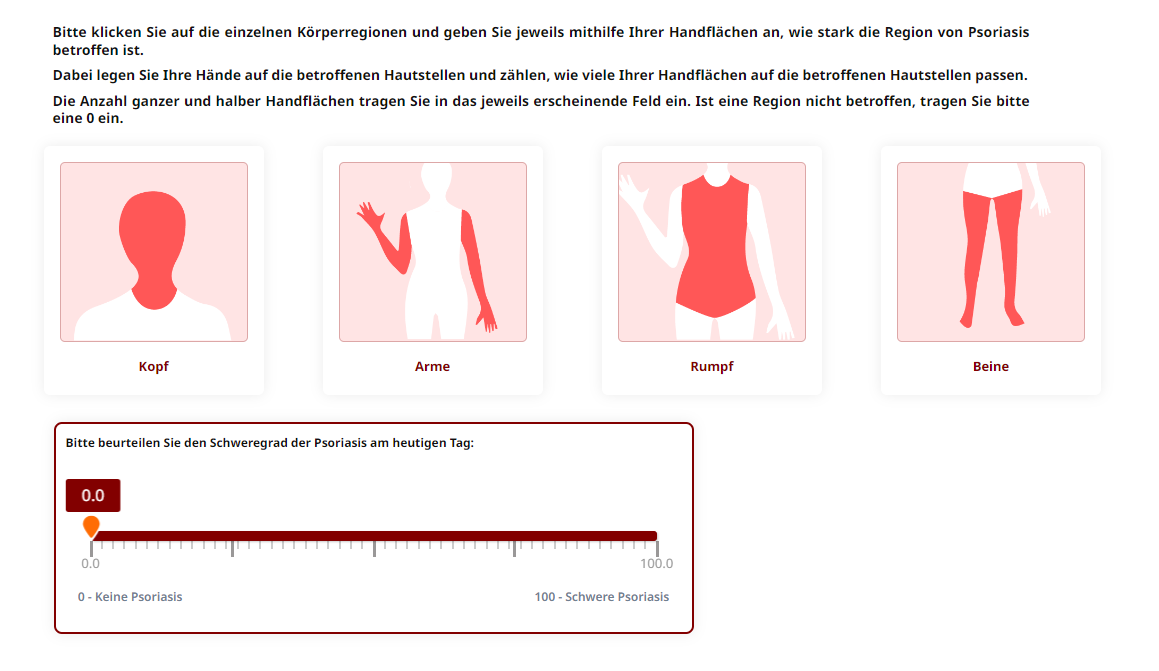
Subsequently, the affected body area is determined. The assessment is carried out using the patient's palm. For each body region (head, trunk, back and legs), the number of palms that fit the affected skin areas is counted. The SA-PASI is derived by a combination of the body area involved and the severity of an average plaque.
The following versions of the eSA-PASI are available
Illustrative PDF
(coming soon)
Original PDF
(coming soon)
Plate XXIII, Psoriasis. Crocker 1896
Credit: Wellcome Library, London
About psoriasis
Psoriasis is a long-lasting autoimmune disease known by its abnormal patches of skin (red, itchy and even scaly).
“Psoriasis is a chronic inflammatory disease, with an incidence in Western industrialized countries of 1.5 % to 3 %. It represents a great burden to the patient due to marked loss of quality of life, therapy-refractory course and frequent side effects of therapy.”
Prof. Dr. med. med. Matthias Augustin
How was the SA-PASI developed?
The SA-PASI was developed at the Bowman Gray School of Medicine of Wake Forest University in the USA. The SA-PASI is a modified version of the original PASI, which requires trained staff for the measurement. Instead of the calculation of the involved Body Surface Area (BSA) by trained personnel, a line-drawing silhouette of the front and back is marked by the patient to estimate the involved body area. In the electronic version of the SA-PASI, the body surface area is calculated using the patient's palm, with one palm representing approximately 1 % of the body surface area. This is done by four body regions (head, trunk, back and legs). Since its original development, the SA-PASI was validated in multiple studies and showed high agreement with the original and well accepted PASI. The electronic version of the SA-PASI is currently validated at the Institute for Health Services Research in Dermatology and Nursing at the University Medical Center Hamburg Eppendorf.
Features
PATIENT-CENTRED
Results from the eSA-PASI provide a patient-orientated analysis of psoriasis severity.
QUICK
Save time with our online tool. Fill in the questionnaire and your eSA-PASI will be calculated automatically.
USER-FRIENDLY
The eSA-PASI is easy and intuitive to use.
VALIDATED
Since its original development, the SA-PASI was validated in multiple studies and showed good agreement with the original and well accepted PASI.
MONITOR PROGRESS
The eSA-PASI can be used to monitor the change in psoriasis severity over the course of treatment.
ACCESSIBLE FROM ALL DEVICES
With DermaValue, your eSA-PASI is always just a few clicks away. The application is available for PCs, tablets, smartphones, and other devices.
References
Validation study:
Feldman, S. R., Fleischer, A. B., Jr, Reboussin, D. M., Rapp, S. R., Exum, M. L., Clark, A. R., & Nurre, L. (1996). The self-administered psoriasis area and severity index is valid and reliable. The Journal of investigative dermatology, 106(1), 183–186. https://doi.org/10.1111/1523-1747.ep12329912
Fleischer AB Jr, Feldman SR, Dekle CL. The SAPASI is valid and responsive to psoriasis disease severity changes in a multi-center clinical trial. J Dermatol. 1999 Apr;26(4):210-5. doi: 10.1111/j.1346-8138.1999.tb03458.x . PMID: 10343464 .
Our other tools
Our Partners





